General requirements bone substitutes - ISO 13175-3
Normative References
ISO 13175-3: Implants for surgery - Calcium phosphates - Part 3: Hydroxyapatite and beta-tricalcium phosphate bone substitutes
This part of ISO 13175 specifies requirements for monophasic hydroxyapatite bone substitutes, monophasic β-tricalcium phosphate bone substitutes and biphasic hydroxyapatite/β-tricalcium phosphate bone substitutes in the form of blocks or granules.
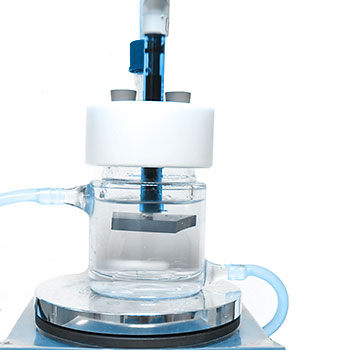
EndoLab® provides highly specialized methods to determine the porosity, micro- and macropores. We do support your test and development needs by our sophisticated methods for dissolution and ph change measurements as well as mechanical testing. As, especially dissolution tests might be time critically in your product development, you might want to benefit from years of experience with this type of testing.