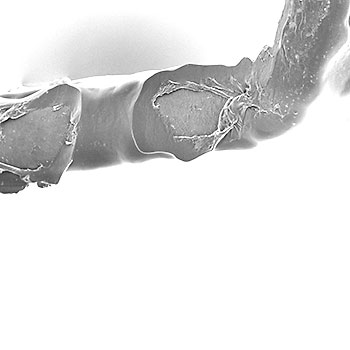
Coating Integrity - ASTM F2743
Normative References
ASTM F2743: Standard Guide for Coating Inspection and Acute Particulate Characterization of Coated Drug-Eluting Vascular Stent.
The test standard describes in-vitro test procedures for assessing the acute coating durability of coated drug-eluting vascular stent systems. Clinical performance (i.e., drug elution) may be affected by coating durability. Balloon-expandable and self-expanding stents are included within the scope. Recommended practices for assessing acute coating durability include baseline (deployment) testing and simulated use testing. We provide several approaches for coating integrity assessment.