Knee Contact Pressure Test - PI-17
Normative References
PI-17: Determination of Total Knee Implant Contact Pressure
ASTM F2083: Standard Specification for Knee Replacement Prosthesis
ASTM F1672: Standard Specification for Resurfacing Patellar Prosthesis
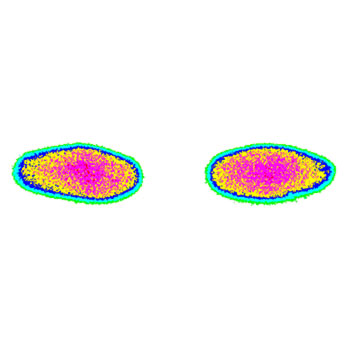
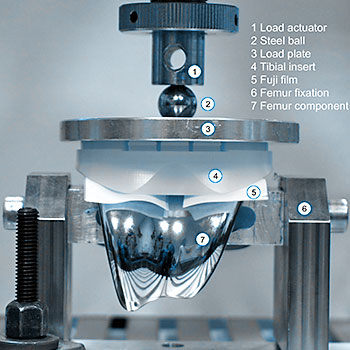
Pressure distribution and pressure levels influence the wear of total knee replacements. Using pressure films, the contact area, as well as the amount of pressure (in MPa), can be determined. We have developed a computerized calibration method to achieve reproducible results while avoiding the disadvantages of pressure films. An individual calibration is performed for every test series. Digital image processing is used to analyse the results.
This test evaluates the pressure distribution and total contact area between the femoral component and tibial insert or between the femoral component and the patella insert of a total knee replacement system under different flexion angles and loads. These results help to develop optimized geometries.