Fusionsimplantate - ASTM F2077
Aktuelle Prüfverfahren
ASTM F2077: Test Methods For Intervertebral Body Fusion Devices
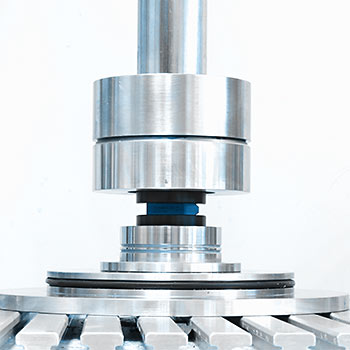
This test method describes the materials and methods for testing of intervertebral body fusion device assemblies, spinal implants designed to promote arthrodesis at a given spinal motion segment. Depending on the intended spinal location, the devices are tested in static and dynamic axial compression, shear compression and/or torsion mode. For testing, the implants are inserted between polyacetal test blocks for dynamic loading or stainless steel blocks for static loading.
The load blocks are prepared in advance to match the geometry of the cage. The load blocks can either be designed based on CAD data of the cages as provided by the client or the cages can be captured by EndoLab® using a high-resolution 3D scanner.