Guide for assessment of hard-on-hard articulation THR devices - ASTM F3018
Normative References
ASTM F3018: Standard Guide for Assessment of Hard-on-Hard Articulation Total Hip Replacement and Hip Resurfacing Arthroplasty Devices
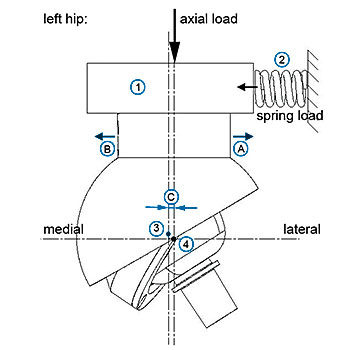
This guide describes material and design recommendations and general test methods for the assessment of implantable devices with metal-on-metal or ceramic-on-ceramic articulations intended to replace a hip joint.
Amongst others, test services evaluating the dimensional properties (such as head-to-cup clearance, articular surface roughness, sphericity, coating characterization) and physical properties (such as wear, friction, impingement, shell/cup deformation, push out strength, strength of ceramic heads) are provided. Appropriate ASTM international or ISO standards are commonly used for these analyses. EndoLab® and its partner laboratories do provide a full test series according to this standard guide.