Vascular implants
Radial Fatigue and Durability - ISO 25539/ASTM F2477Radial Fatigue and Durability - ISO 25539/ASTM F2477
Axial, Bending and Torsional Durability Testing - ASTM F2942Axial, Bending and Torsional Durability Testing - ASTM F2942
Resistance to Perpendicularly-applied Load - ISO 25539Resistance to Perpendicularly-applied Load - ISO 25539
Crush Resistance and Radial Force - ISO 25539/ASTM F3067Crush Resistance and Radial Force - ISO 25539/ASTM F3067
Kink Resistance (Flexibility) - ISO 25539/ASTM F3505Kink Resistance (Flexibility) - ISO 25539/ASTM F3505
Stent-free Surface Area and Stent Outer Surface Area - ISO 25539/ASTM F2081Stent-free Surface Area and Stent Outer Surface Area - ISO 25539/ASTM F2081
Dimensional Verification - ISO 25539/ASTM F2081Dimensional Verification - ISO 25539/ASTM F2081
Local Compression - ISO 25539Local Compression - ISO 25539
Stent System Three-point Bending - ASTM F2606Stent System Three-point Bending - ASTM F2606
Corrosion Test of Small Implants - ASTM F2129Corrosion Test of Small Implants - ASTM F2129
Galvanic Corrosion - ASTM F3044Galvanic Corrosion - ASTM F3044
Balloon Deflation Time - ISO 25539Balloon Deflation Time - ISO 25539
Balloon Rated Burst Pressure (RBP) - ISO 25539Balloon Rated Burst Pressure (RBP) - ISO 25539
Balloon Rated Fatigue - ISO 25539Balloon Rated Fatigue - ISO 25539
Dogboning - ISO 25539Dogboning - ISO 25539
Dimensional Verification - ISO 25539/ASTM F2081Dimensional Verification - ISO 25539/ASTM F2081
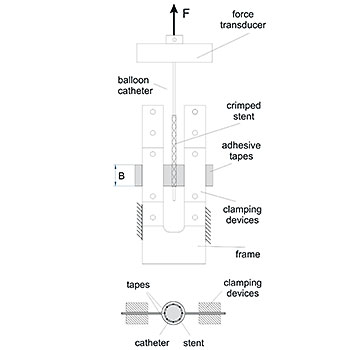
Dislodgement Force - ISO 25539/ASTM F2394Dislodgement Force - ISO 25539/ASTM F2394
Force to Deploy - ISO 25539Force to Deploy - ISO 25539
Coating Integrity - ASTM F2743Coating Integrity - ASTM F2743
Profile Effect / Flaring - ISO 25539Profile Effect / Flaring - ISO 25539
Simulated Use - ISO 25539Simulated Use - ISO 25539
Tensile Bond Strength - ISO 25539Tensile Bond Strength - ISO 25539
Torsional Bond Strength - ISO 25539Torsional Bond Strength - ISO 25539
Stent Diameter to Balloon Inflation Pressure - ISO 25539Stent Diameter to Balloon Inflation Pressure - ISO 25539
Stent Length - ISO 25539/ASTM F2081Stent Length - ISO 25539/ASTM F2081
Recoil - ISO 25539/ASTM F2079Recoil - ISO 25539/ASTM F2079
Stent System Three-point Bending - ASTM F2606Stent System Three-point Bending - ASTM F2606
Sterile and Single-use Catheters: General Requirements - ISO 10555-1Sterile and Single-use Catheters: General Requirements - ISO 10555-1
Central Venous Catheters - ISO 10555-3Central Venous Catheters - ISO 10555-3
Balloon Dilation Catheters - ISO 10555-4Balloon Dilation Catheters - ISO 10555-4
Over-needle peripheral catheters - ISO 10555-5Over-needle peripheral catheters - ISO 10555-5
Small-bore Connectors Test Methods - ISO 80369-20Small-bore Connectors Test Methods - ISO 80369-20
Dislodgement Force - ISO 25539/ASTM F2394
Normative References
ISO 25539‐1: Cardiovascular implants - Endovascular devices - Part 1: Endovascular prostheses
ISO 25539‐2: Cardiovascular implants - Endovascular devices - Part 2: Vascular stents
ASTM F2394: Measuring Securement of Balloon Expandable Vascular Stent Mounted on Delivery System
For the pre-mounted balloon-expandable implant, EndoLab® GmbH determines the force required to pull off the crimped implant from the non-expanded balloon. This test is conducted at the proximal and distal ends of the implant.