Radial Fatigue and Durability - ISO 25539/ASTM F2477
Normative References
ISO 25539‐1: Cardiovascular implants - Endovascular devices - Part 1: Endovascular prostheses
ISO 25539‐2: Cardiovascular implants - Endovascular devices - Part 2: Vascular stents
ISO 7198: Cardiovascular implants and extracorporeal systems — Vascular prostheses — Tubular vascular grafts and vascular patches
ASTM F2477: Standard Test Methods for in vitro Pulsatile Durability Testing of Vascular Stents.
The fatigue life of vascular prostheses, such as coronary and peripheral stents and bifurcated devices, are verified under simulated physiological loading conditions.
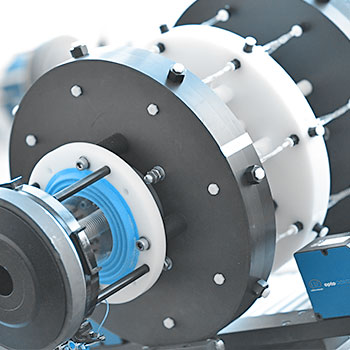
Our test setup consists of an array of mock vessels pulsated by electro-dynamic motors. This simulates physiological loading conditions on the implanted stents.
The decrease and increase of silicone vessel diameter define the loading and unloading of the stent. This value is used for digital closed-loop control of the test system and allows for high precision control even when testing extremely small or large (peripheral) vascular implants. A contactless diameter scanning system continuously scans the actual outer diameter of the mock vessel. The corresponding change in the inner diameter is evaluated by analytical means.
According to ASTM F2477, the typical duration of this test is 10 years of equivalent use (at 72 beats per minute) or at least 380 million cycles. Most fatigue tests operate at frequencies between 20 and 60 Hz. Customized solutions are possible by request.