Dauerschwingversuch Tibiaplateau - ISO 14879-1/ ASTM F1800
Aktuelle Prüfverfahren
ISO 14879-1: Implants for surgery - Total knee-joint prostheses - Part 1: Determination of endurance properties of knee tibial trays
ASTM F1800: Standard Practice for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements
ISO 21536: Non-active surgical implants - Joint replacement implants - Specific requirements for knee-joint replacement implants
ASTM F2083: Standard Specification for Knee Replacement Prosthesis
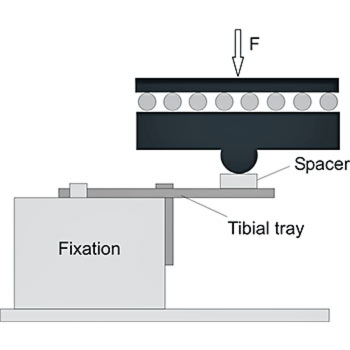
The tibial trays in a total knee endoprosthesis are subjected to fatigue stress. The worst-case condition occurs if medial loading is applied without bone support. To simulate this worst-case scenario, we fixate the tibial trays unilaterally using a metal block. To avoid restraint forces, EndoLab® uses a load application mechanism allowing free movement on the horizontal plane.
Safety against fracture is determined up to 10 million cycles, testing five implants at 900 N maximum load (ISO requirements). Due to clinical fractures of implants that have passed fatigue testing at 900 N, EndoLab® recommends testing at a higher load level of 2,000 N. In contrast, tibial tray designs with short pegs did fail at 900 N, with no clinical evidence of fatigue failure.