Shoulder implants
Reverse shoulder implant wear test - PI-61
Normative References
PI-61: Reverse Shoulder System Wear Test
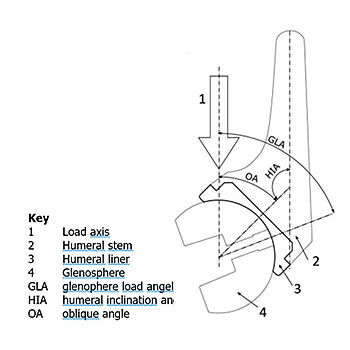
The test parameters for reverse shoulder replacement systems are based on a wear study conducted by Kohut et al [1]. For in vitro testing the humeral component is positioned superior to the glenosphere component and the joint load is applied vertically. The angles between the component axes and the load line at the reference position are recommended to be chosen based on a study of Terrier et al [2].
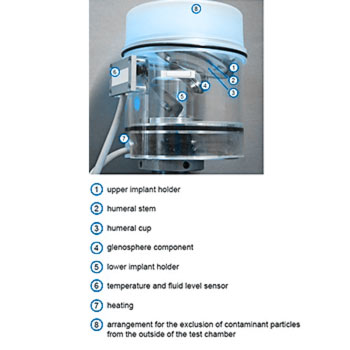
The EndoLab® hip joint simulator is used for the testing. Following motions are applied simultaneously to the implant system: Abduction/adduction, flexion/extension and internal/external rotation. A sinusoidal joint load ranging between 250 N and 1000 N is applied. The specimens are assembled under laminar flow conditions and sealed by capsulated single test chambers to exclude contamination. The temperature of the test fluid as well as the fluid level are controlled throughout testing. The wear of the components is determined gravimetrically. The wear test is conducted for 5 million cycles.
[1] Georges Kohut, Frank Dallmann, Ulrich Irlenbusch: Wear-induced loss of mass in reversed total shoulder arthroplasty with conventional and inverted bearing materials. Journal of Biomechanics 45 (2012) 469-473
[2] A. Terrier; A. Reist, F. Merlini, A. Farron: Simulated joint and muscle forces in reversed and anatomic shoulder prostheses. The Journal of Bone & Joint Surgery. Vo. 90-B, no. 6, June 2008