Knee implants
all procedures accredited
Knee wear test - ISO 14243-1/3
Normative References
ISO 14243-1: Implants for surgery - Wear of total knee joint prostheses
Part 1: Loading and displacement parameters for wear-testing machines with load control and corresponding environmental conditions for tests.
ISO 14243-2: Implants for surgery - Wear of total knee joint prostheses Part 2: Methods of measurement.
ISO 14243-3: Implants for surgery - Wear of total knee joint prostheses, Part 3: Loading and displacement parameters for wear testing machines with displacement control and corresponding environmental conditions for test.
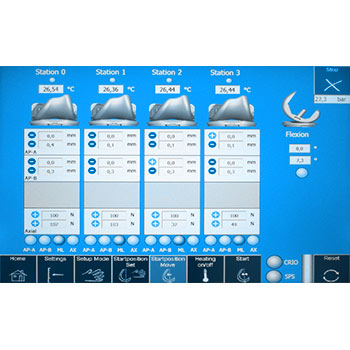
Wear of total knee endoprostheses as well as the implant kinematics can be determined using a total knee simulator. EndoLab® has developed a simulator that allows either a testing in force control (ISO 14243-1) or in displacement control (ISO 14243-3). Due to the servohydraulic actuators, excellent machine accuracy has been reached. Please refer the support section to see the simulator moving. Simultaneous testing of three (plus soak control) specimens enables rapid and economic developments. Routine testing is performed using calf serum as a test fluid. In general, the test is stopped after 5 million cycles. Wear is determined by weight loss and/or geometric measurements.
To see the simulator moving open video on the external service provider YouTube .
The wear of the components is determined gravimetrically, particle analysis can be performed. Calf serum with a protein content of 20 g/l is used for the tests.