Hip implants
all procedures accredited
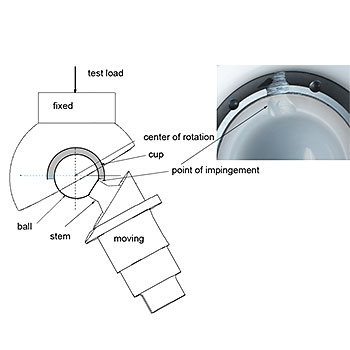
Impingement Test - ASTM F2582
Normative References
ASTM F2582: Standard test method for impingement of acetabular prostheses
This test method covers a procedure to simulate dynamic impingement between femoral and acetabular components in a hip replacement, the subsequent qualitative assessment of damage modes, and, if necessary, quantitative assessment of changes in modular component.
The EndoLab® hip joint simulator is used for this type of testing. According to ASTM F2582 all UHMWPE acetabular components have to be artificially aged prior to testing (see ASTM F2003 ). All three in-vivo angular displacements (flexion/extension, abduction/adduction and internal/external rotation) are simulated and a constant joint reaction force is applied. The specimens are inspected every 200,000 cycles up to 1.0 million cycles are reached or failure of the system.
As this test is intended to generate damage at the contact area, the test standard defines acceptable and non-acceptable damage modes.