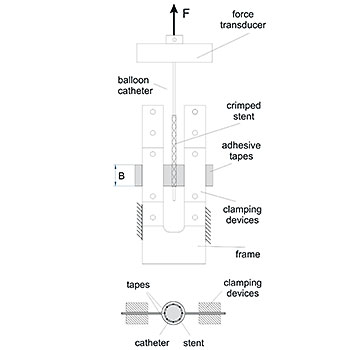
Dislodgement Force - ISO 25539/ASTM F2394
Normative References
ISO 25539‐1: Cardiovascular implants - Endovascular devices - Part 1: Endovascular prostheses
ISO 25539‐2: Cardiovascular implants - Endovascular devices - Part 2: Vascular stents
ASTM F2394: Measuring Securement of Balloon Expandable Vascular Stent Mounted on Delivery System
For the pre-mounted balloon-expandable implant, EndoLab® GmbH determines the force required to pull off the crimped implant from the non-expanded balloon. This test is conducted at the proximal and distal ends of the implant.